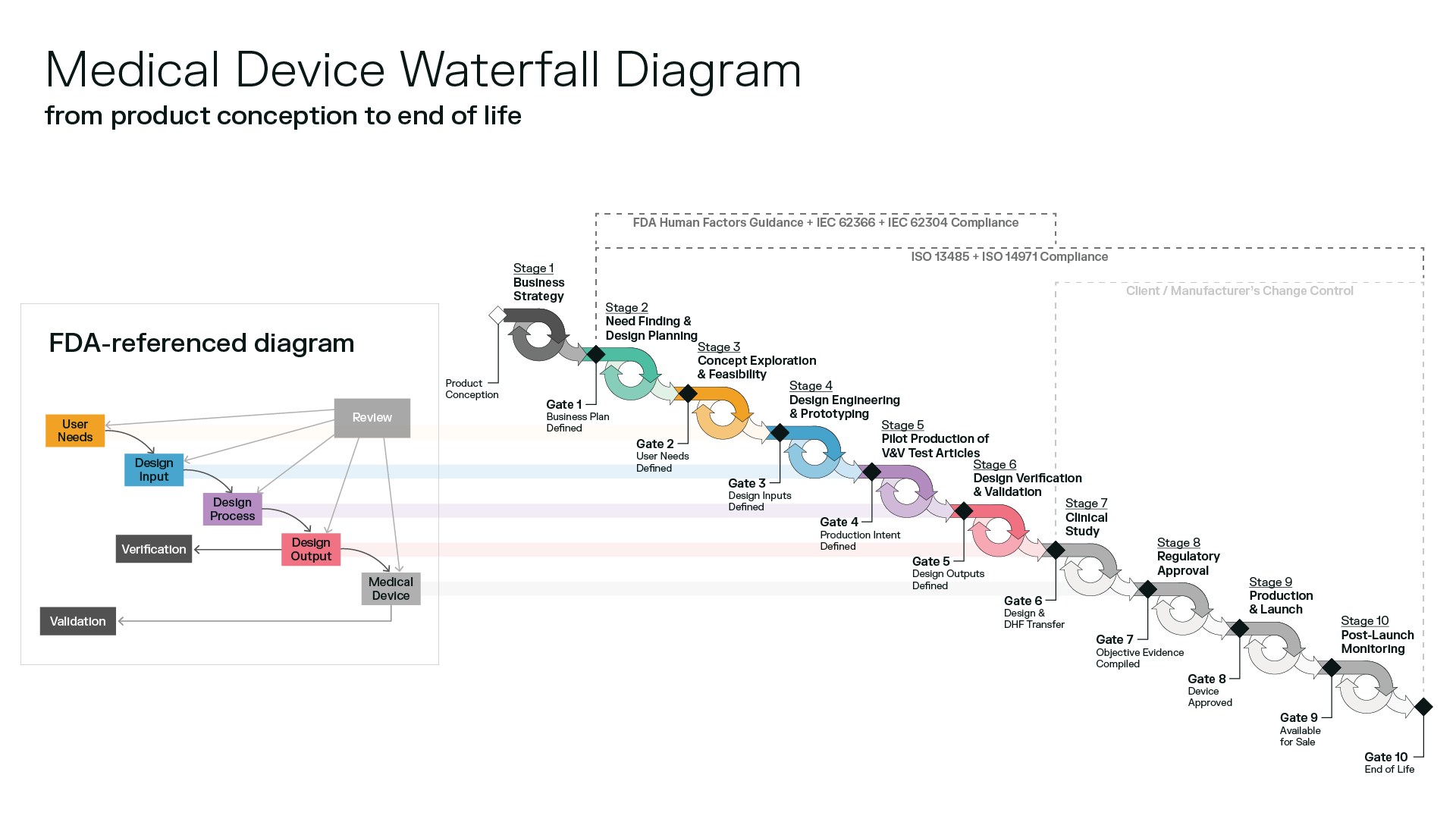
The Medical Device Design Process
Having trouble deciphering the FDA waterfall diagram process for medical device design and development? You’re not the only one.
The Medical Device Design Process
Let's build a breakthrough medical device.
Biggest Innovations in 2022 That Influenced Product Design
A coffee pod system without a plastic pod. Headphones that read your mind. A touch-sensitive prosthetic hand. Check out the biggest innovations to influence product design in 2022.
Read more
5 Groundbreaking Health Innovations Powered by Data and Machine Learning
Advances in data science and machine learning are transforming healthcare and public safety. Countless lives will benefit. Here are five inspiring examples.
Read more
What Mini Electronics Are Inside Tiny Wearables?
We opened up wearables from leading companies to see how so much was packed into something so little.
Read more
Why IoT Data Collection Is Essential To Get Right
IoT data collection impacts data deployment, sensor selection, security, and more. It's at the core of every smart product's user experience — and success.
Read more
Innovative Technology To Combat Climate Change: Our 7 Favorite Solutions
A list of emerging, innovative technology being developed to reduce carbon, geoengineer the environment, and improve battery efficiency and cooling tech.
Read more
Our DIY Split-Flap Display: An Adventure in Inefficient Nostalgia
We weren't setting out to invent something radically different, but we wanted to prove to ourselves that we could make our own split-flap display.
Read more